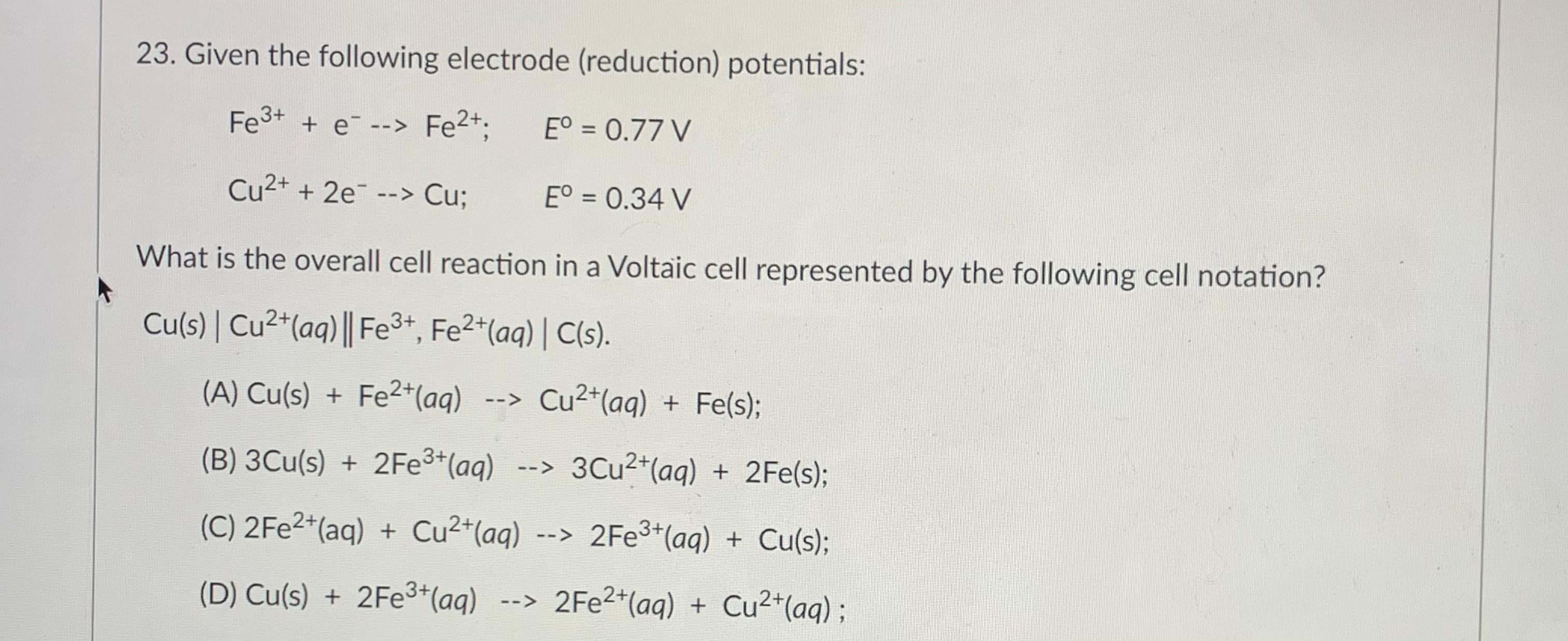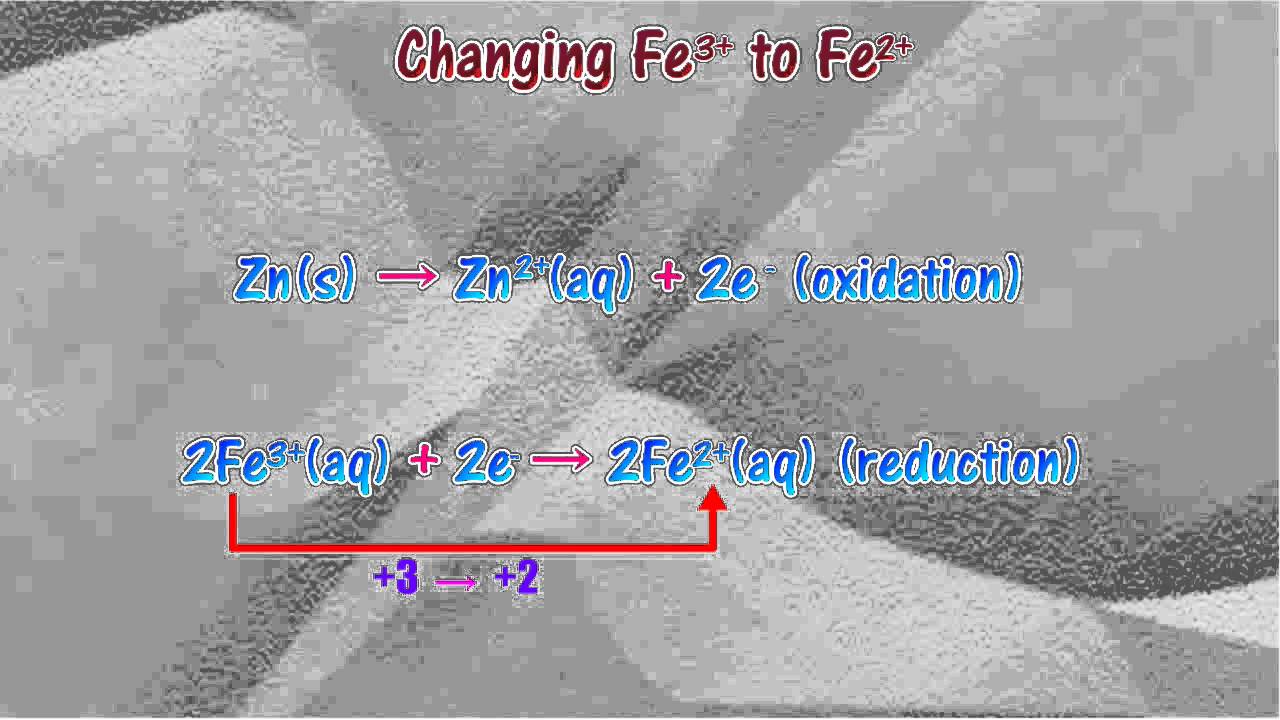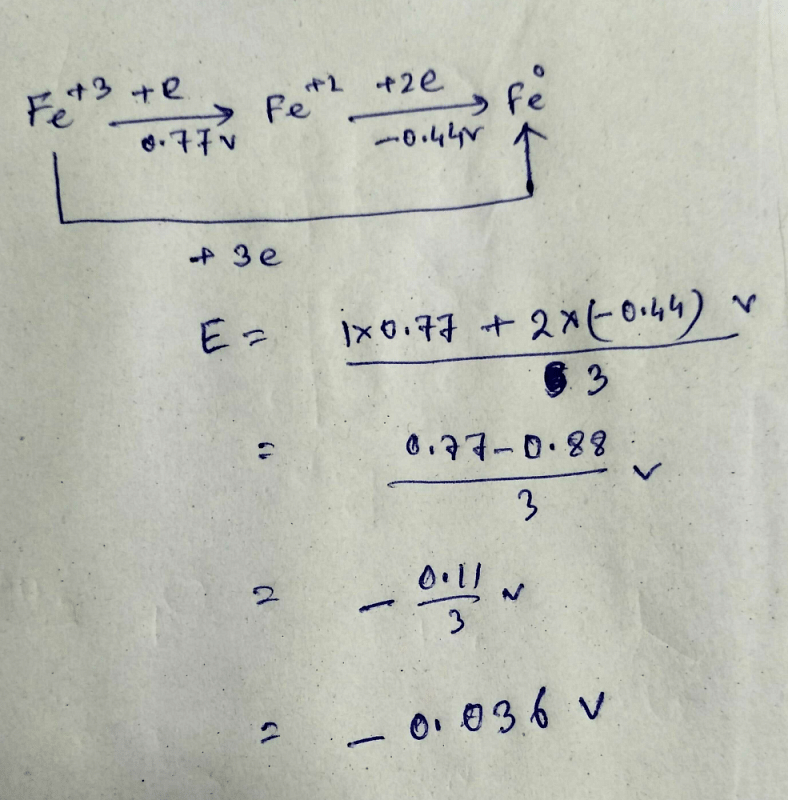
The standard reduction potentials of the Fe3+/Fe2+ and Fe2+/Fe couples are 0.77 and 0.44 V, respectively. The standard reduction potential (in V) for the Fe3+/Fe couple is _______Correct answer is '-0.03'. Can
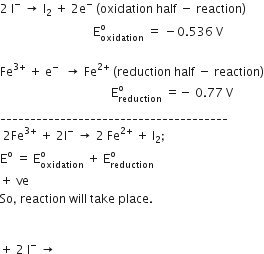
A solution contains Fe2+ Fe3+, and I- ions. This solution was treated with iodine at 35o C.Eo for Fe3+/ Fe2+ is +0.77 V and Eo for I2/ 2 I- = 0.536 V.
Standard electrode potentials are Fe2+/Fe; E° = 0.44 volts Fe3+/Fe2+; E° = 0.77 volts Fe2+, Fe3+ and Fe blocks are kept together, then (1) Fe2+ increases (2) Fe3+ decreases (3) Fe2+/Fe3+ remains unchanged (4) Fe2+ decreases

Given electrode potentials are : Fe^3 + + e^- → Fe^2 + ; E^ = 0.771 V I2 + 2e^- → 2I^ ; E^ = 0.536 V Find the E^ cell for the cell reaction: 2Fe^3 + + 2I^ → 2Fe^2 + + I2 is :

Fe3+/Fe2+ ratio in slag as a function of time at 1573 K (1300 °C) and... | Download Scientific Diagram

Inhibition of Fe2+- and Fe3+- induced hydroxyl radical production by the iron-chelating drug deferiprone - ScienceDirect