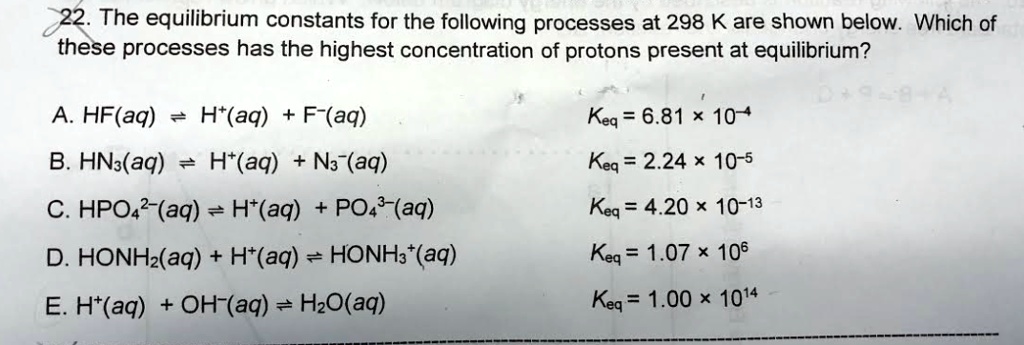
SOLVED: Which of the following reactions would you predict will go closest to completion; giving relatively large concentration of products? cO(g) Clz(g) cOClzug) Keq-22 PCIgug) Cl2(g) pcisig) Keq= 2.9X10-2 CO(g) Clz(g) cociz(e)

SOLVED: If the equilibrium constant (Keq) of a reaction i5 0.5 then which of the following that must be true? Gibbs free energy (G) is negative The reaction will have an early

Equilibrium Equations- Using Keq To Solve Problems | Part One Chemistry 30 Video Tutorial Solving Equilibrium Expressions: Using Keq To Solve Problems #EquilibriumExpressions #Chemistry #VideoTutorial... | By TutorTag | Facebook
![OneClass: Calucate average Keq. calculate equilibrum of (FeNCS2+] [fe3+] [SCN -] Desk Laboratory Inst... OneClass: Calucate average Keq. calculate equilibrum of (FeNCS2+] [fe3+] [SCN -] Desk Laboratory Inst...](https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/116/11695188.jpeg)
OneClass: Calucate average Keq. calculate equilibrum of (FeNCS2+] [fe3+] [SCN -] Desk Laboratory Inst...
49.Given that the equilibrium constant for the reaction3(g)as a value of 278 at a particular temperature.What is the value of the equilibrium constant forthe following reaction at the same temperature?AIPMT 20122SOsg)SO29)22(1) 1.8