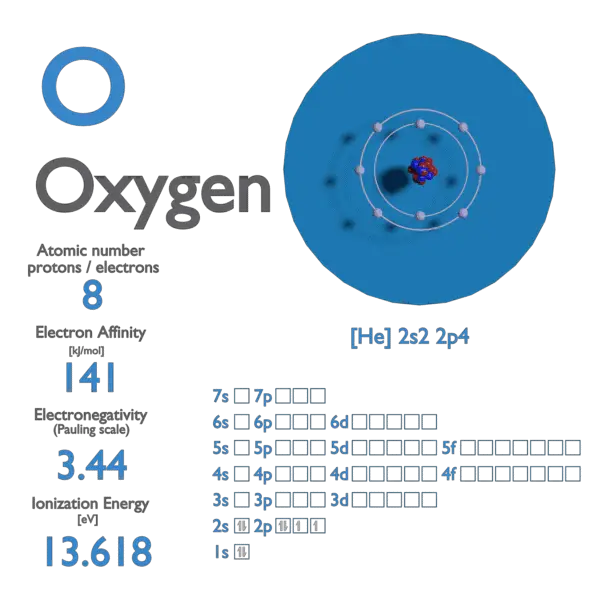
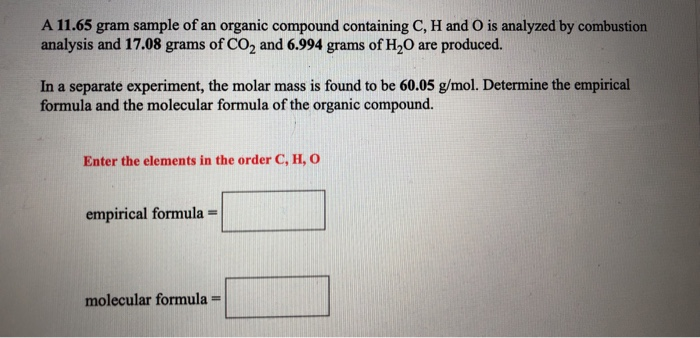
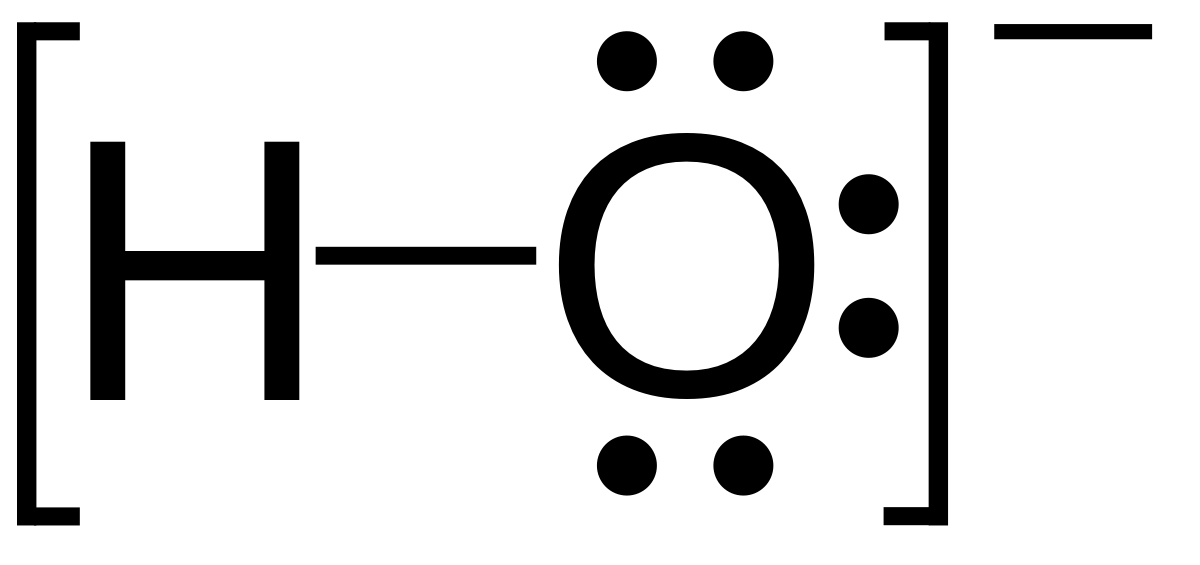
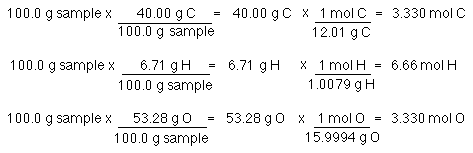SOLVED: Determine the number of moles of oxygen atoms in each sample. a. 4.88 mol H2O2 b. 2.15 mol N2O c. 0.0237 mol H2CO3 d. 24.1 mol CO2

Mole Concept and Chemical Calculations: Difference between Relative Atomic Mass, Relative Molecular Mass, Relative Formula Mass and Molar Mass

Moles”. “Moles” MOLE is a way of translating between grams and amu's a mole is a number that helps us translate between the atomic world and our. - ppt download
What is the molecular formula of a compound with a percent composition of 49.47% C, 5.201% H, 28.84% N, and 16.48% O, and a molecular mass of 194.2 g/ mol? - Quora